Zinc oxide is widely used in rubber, paint, ceramics, chemical, pharmaceutical, glass and electronics industries. With the rapid development of industry, the domestic demand for zinc oxide is increasing. The use of low-grade zinc-containing materials to produce active zinc oxide not only makes full use of zinc resources, but also reduces production costs. Therefore, the research field is currently very active, comprehensively utilizing low-grade oxidized ore, sub-oxidized ore, zinc slag, soot, etc. Research has gradually attracted the attention of enterprises. Soot lead, an intermediate product of metallurgical processes zinc is volatilized from the rotary kiln, depleted of zinc lead blast furnace slag and other materials generated by its complex composition, in addition to zinc, also contain lead more arsenic, 锑 and other impurities. Due to its difficulty in processing and high cost, it cannot be directly used as a raw material for wet zinc smelting. However, since it has a high zinc content and is easily leached into a human solution, it can be used as a zinc oxide powder product.
The existing methods for treating soot are acid leaching method and ammonia compounding method. The acid leaching method uses zinc oxide or zinc ore as raw material, and reacts with dilute acid to obtain zinc oxide by impurity removal, neutralization, drying and calcination. The method has large impurity removal, complicated process, high cost, large amount of waste water, difficult treatment and pollution to the environment. The ammonia compounding method is a process of using zinc oxide or desulfurized zinc catalyst calcined material as raw material, ammonia water-ammonium bicarbonate solution as leaching agent, leaching, decontamination purification, steaming ammonia zirconium, washing and drying, and calcination. Active zinc oxide is produced. The method has less investment in equipment and less impurities, but the process is long, and it is only suitable for materials rich in ZnO. If the material contains a certain amount of ZnS, the method cannot be directly used, and the oxidant is used to pre-treat the soot and zinc sulfide. Converted to zinc oxide. Therefore, it has been experimentally studied to pretreat soot with hydrogen peroxide and then leaching with sodium hydroxide solution to obtain a zinc oxide powder product.
First, the test part
(1) Test materials
The test soot was taken from a factory in Guangxi with a particle size of 65-76 μm. The main chemical compositions are shown in Table 1.
Table 1 Chemical composition of soot
Zn | Pb | Fe | As | Cd | Sb | Si | S |
49.86 | 26.89 | 0.75 | 0.80 | 0.03 | 0.11 | 0.5 | 1.81 |
(2) Test methods
Firstly, the dry soot is prepared into a soot slurry with a liquid solid mass ratio of 1:1, and then pretreated with 3% hydrogen peroxide for a certain period of time, and then added with a certain amount of sodium hydroxide solution at a certain temperature, stirred and leached, and then centrifuged to filtrate. After appropriate dilution and stirring for a period of time, the mixture is centrifuged again, and the secondary filtrate is returned as a leaching agent, and the filter cake is zinc hydroxide. After washing, drying and calcining, pure zinc oxide powder is obtained.
Second, the results and discussion
(1) Effect of volume fraction of hydrogen peroxide and pretreatment temperature on zinc leaching rate
At different temperatures, add a certain amount of hydrogen peroxide to 100g of soot slurry (liquid solid mass ratio 1:1), stir for 60min, and then immerse in 3mol/L sodium hydroxide solution for 2h at 60°C to observe the volume fraction of hydrogen peroxide. And the effect of pretreatment temperature on zinc leaching rate in soot, the results are shown in Table 2.
Table 2 Effect of volume fraction of hydrogen peroxide and pretreatment temperature on zinc leaching rate
Test number | Hydrogen peroxide volume fraction /% | Pretreatment temperature / °C | Zinc leaching rate /% |
1 | 1 | 25 | 38.24 |
2 | 3 | 25 | 55.67 |
3 | 5 | 25 | 56.71 |
4 | 1 | 40 | 40.33 |
5 | 3 | 40 | 60.01 |
6 | 5 | 40 | 61.24 |
It can be seen from Table 2 that the volume fraction of hydrogen peroxide increases and the leaching rate of zinc increases. At 25 °C, when the volume fraction of hydrogen peroxide increases from 1% to 3%, the zinc leaching rate increases by 17%; the volume fraction of hydrogen peroxide is from 3%. When the temperature is increased to 5%, the zinc leaching rate is only increased by 1%; when the temperature is raised to 40 °C, the volume fraction of hydrogen peroxide is increased from 1% to 3%, the zinc leaching rate is increased by nearly 20%, and the SO of zinc oxide is adsorbed. 2 is oxidized by hydrogen peroxide to zinc sulfate, which does not pollute the environment. It can be considered that the temperature has little effect on the zinc leaching rate, and the volume fraction of hydrogen peroxide is 3%.
(2) Effect of temperature on zinc leaching rate
The soot was pretreated with 3% hydrogen peroxide at 25 ° C, and then leached with a 3 mol/L sodium hydroxide solution at different temperatures for 1.5 h. The test results are shown in Figure 1.

Fig.1 The leaching temperature on the rate of zinc diffusion in soot
It can be seen from Figure 1 that as the leaching temperature increases, the zinc leaching rate increases linearly. At room temperature, the zinc leaching rate is only 30.22%, and when the temperature is raised to 95 °C, the zinc leaching rate reaches 89.31%. Considering comprehensively, the leaching temperature is preferably 85 °C.
(3) The effect of sodium hydroxide concentration on zinc leaching rate
The soot was pretreated with 3% hydrogen peroxide at 25 ° C and then leached with different concentrations of sodium hydroxide solution at 85 ° C for 1.5 h. The test results are shown in Figure 2.

Figure 2 Effect of sodium hydroxide concentration on zinc leaching rate
It can be seen from Fig. 2 that as the concentration of sodium hydroxide increases, the zinc leaching rate increases, especially the concentration of sodium hydroxide increases from 2 mol/L to 5 mol/L, and the zinc leaching rate increases by 46.54% to 97%. This is due to the reaction of zinc and alkali in the soot to form sodium zincate into the solution:
2 NaOH + ZnO = Na 2 ZnO 2 + H 2 O.
However, when the concentration of sodium hydroxide is increased to 6 mol/L, the zinc leaching rate is only increased by 0.52%, which cannot reach 100%. This may be because the zinc in the soot is wrapped and cannot be contacted with the alkali.
(4) Effect of leaching time on zinc leaching rate
The soot was pretreated with 3% hydrogen peroxide at 25 ° C, and then leached with a 3 mol/L sodium hydroxide solution at 85 ° C to investigate the effect of leaching time on zinc leaching rate. The result is shown in Figure 3.

Figure 3 Effect of leaching time on zinc leaching rate
It can be seen from Fig. 3 that as the reaction time increases, the zinc leaching rate increases. After leaching for 0.5 to 1.5 hours, the zinc leaching rate increased from 73.81% to 96.92%; however, after 1.5 hours of leaching, the zinc leaching rate increased slowly. Therefore, the leaching time is preferably 1.5h.
(5) Verification test
The soot was pretreated with 3% hydrogen peroxide at 25 ° C, and then leached with a 5 mol / L sodium hydroxide solution at 85 ° C for 1.5 h.
The zinc leaching rate and the mass fraction of zinc and lead in the leaching slag are shown in Table 3.
Table 3 Test results of alkali leaching soot verification test
Test number | Zinc leaching rate /% | ωB/% in leaching residue | ||
Zn | Pb | As | ||
1 | 96.92 | 3.91 | 45.33 | 0.02 |
2 | 97.03 | 3.42 | 45.20 | <0.01 |
3 | 96.98 | 4.01 | 45.28 | 0.01 |
4 | 97.13 | 3.66 | 46.21 | <0.01 |
It can be seen from Table 3 that under the comprehensive test conditions, the zinc leaching rate is about 97%, the zinc content in the leaching slag is between 3% and 4%, and the lead mass fraction is about 45%, which contains almost no As. Leaching slag can enter the lead system to extract lead and realize comprehensive utilization of resources.
(6) Preparation of zinc oxide
The alkali leaching solution was cooled to 25 ° C, diluted 1 time, stirred for 0.5 h, then centrifuged, and the filter cake was dried, and the zinc hydroxide precipitation rate was 72.3%. The XRD analysis of the precipitate showed that the phase composition was mainly ZnO. The chemical analysis showed that the mass fraction of ZnO was 99.58% and the mass fraction of Pb0 was 0.12%, which basically met the requirements of the direct method.
Third, the conclusion
The zinc-containing soot is pre-oxidized by hydrogen peroxide at room temperature and then leached with sodium hydroxide solution, 97% of the zinc can be transferred into the solution, and then precipitated, filtered and dried to obtain zinc oxide powder. The purity of the ZnO powder obtained by the method is high, which provides an effective way to make full use of the zinc-containing soot.
"Water treatment" refers to the process of removing some harmful substances in water that are not necessary for production and life through physical, chemical and biological means.It is the process of settling, filtering, coagulation, flocculation, corrosion inhibition and scale inhibition of water for specific purposes.
Common Water Treatment Products are as follows:
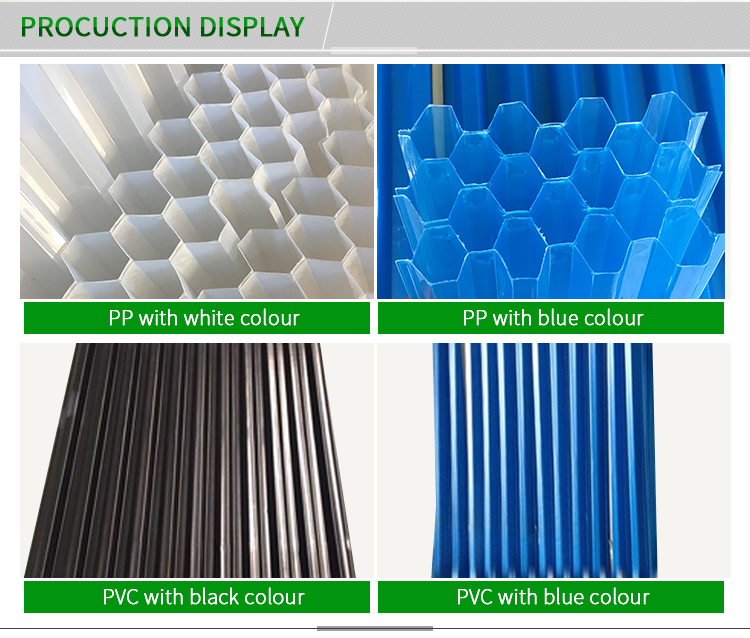
1.Tube Settler:
Also named Hexagonal honeycomb packing.The hexagon honeycomb packing lamella plate clarifier tube settler is very suitable in all different clarifiers and removing sand. It is regarded as universal water treatment equipment in water supply and drainage engineering. It has wide application, high handling efficiency, small area, etc.
It is suitable in removing sand in inlet, industry and drinking water precipitation, separation in oil & water.
The material of tube settlers are PP and PVC.And the size also could be customized according to your requirements.
2.Mbbr Media:
Bio filter media which is used in MBBR Process, which is one of methods used in the reduction of the nitrogen from the waste water efficiently. It mainly uses high surface area media to provide waste water treatment at a faster rate. It is backed with hi-tech aeration systems and has low energy requirement.
MBBR Media is a new kind of suspension carriers. It modified polymeric material with the promoted additions of bio-enzyme, it improves the enzymatic catalysis, so it possess excellences of larger specific surface, being easy to multiply bio film, unease to fall off, high denitrification, good hydrophile, high biological activity and so on.
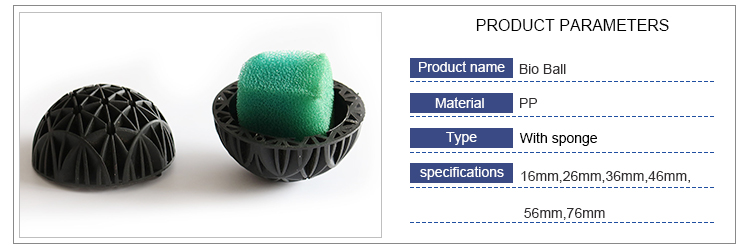
3. Bio Ball :
The bio ball is idea for external and pressurized filters in aquarium tank, pond filter and fish farm water treatment.Bio balls provide large living area for beneficial bacterial to thrive , placed in a filter pond or tank, they efficiently reduce ammonia and nitrite to help maintain healthy aquarium tank, pond or fish farm water conditions and over all well -being of pond or fish farm fish and plants. We also can design special demand as you need.

4.Fine Bubble Diffuser/Fine Bubble Aerator:
Fine Bubble Diffuser is a necessary equipment for air blast aeration aeration.The selection of aeration equipment not only affects the effect of sewage biochemical treatment, but also affects the occupation, investment and operation cost of sewage farm.The main types of micro-hole aerators are: diaphragm type micro hole aerator, pipe aerator, disk aerator, micro-hole ceramic aerator, etc.
Diaphragm type micro orifice aerator is the most new type of aeration device. It has small aeration bubble diameter, small gas-liquid interface diameter, large gas-liquid boundary area, and uniform bubble diffusion, which will not cause hole blockage and strong corrosion resistance.

Water Treatment Products
Water Treatment Products,Water Purification Systems,Water Treatment Systems,Boiler Water Treatment
Hebei Long Zhuo Trade Co., Ltd. , https://www.hblongzhuo.com
